3. Chemical Reactions
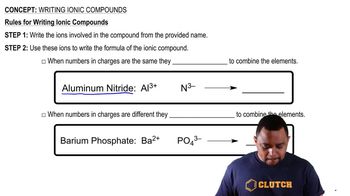
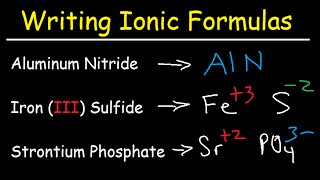
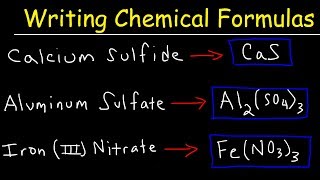
Writing Ionic Compounds
3. Chemical Reactions
Writing Ionic Compounds
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
Provide the molecular formula for the following compound:Copper (I) nitrate
2003views8rank2comments - Multiple Choice
Provide the molecular formula for the following compound:Sodium dichromate
3660views25rank2comments - Multiple ChoiceWhich of the following statements is false?884views
- Open Question
Predict the chemical formula for the ionic compound formed by the elements Ca and Br.
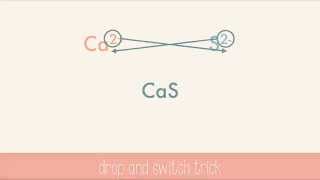
751views - Open QuestionWhen calcium reacts with sulfur the compound formed is1254views
- Open Question
When magnesium (Mg2+) and fluorine (F1-) combine in an ionic bond, the resulting formula will be
1019views - Open Question
What is the formula of a compound that contains Na+ and PO43- ions?
855views - Multiple ChoiceNa reacts with element X to form an ionic compound with the formula Na₃X. Based on this information, what is the likely formula for the ionic compound formed between Al and X?375views