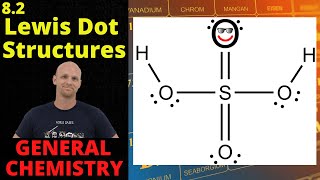
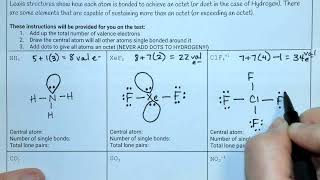
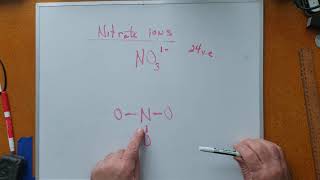11. Bonding & Molecular Structure
Lewis Dot Structures: Ions
11. Bonding & Molecular Structure
Lewis Dot Structures: Ions
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
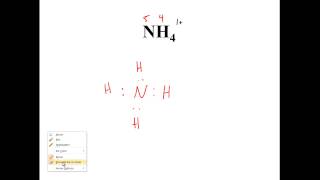
Draw the Lewis Dot Structure for the following cation:NH4+.
10395views19rank - Multiple Choice
Draw the Lewis Dot Structure for potassium hypochlorite, KClO.
2561views9rank1comments - Open Question
Draw the Lewis Dot Structure for calcium cyanide, Ca(CN)2.
2900views15rank5comments - Multiple Choice
Determine the Lewis Dot Structure for the following ion:O22–.
3975views18rank1comments - Multiple Choice
Determine the Lewis Dot Structure for the following ion:SCl42+.
3035views11rank - Open QuestionWhich of the following correctly represent the placement of electrons around each atom?1157views
- Open Question
The lewis structures of methane, the carbonate ion, carbon dioxide, and the sulfite ion are given.
967views - Open Question
Draw the Lewis structure for PCl6−, then answer the following questions:
1011views