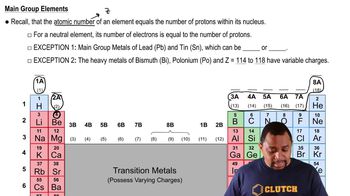
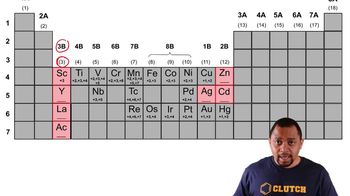
2. Atoms & Elements
Periodic Table: Charges
2. Atoms & Elements
Periodic Table: Charges
Showing 6 of 6 videos
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
Which element possesses a -2 charge when it combines with other elements?
1342views14rank - Multiple Choice
What is the likely charge of the element with an atomic number of 47?
1790views12rank - Multiple Choice
How many electrons would the cadmium ion possess?
2867views18rank - Open Question
What is the number of valence electrons in Cadmium (Cd)? Is it 1, 2, 10, or 12?
782views - Open QuestionPredict the charge of the ion formed by each of the following elements.876views
- Open QuestionIf the following elements were to form ions, what is the expectant charge?1028views
- Open Question
What is the name of the aluminum ion? Al–1 Al+2 Al–3 Al+3?
1006views - Multiple ChoiceDo elements in Group 2 tend to lose electrons to form cations with a charge of +2? Because?372views