1. Intro to General Chemistry
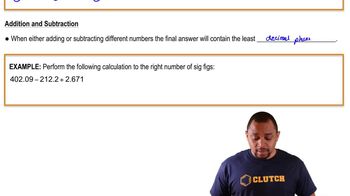
Significant Figures: In Calculations
1. Intro to General Chemistry
Significant Figures: In Calculations
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
What answer should be reported, with the correct number of significant figures, for the following calculation?
3204views36rank7comments - Multiple Choice
Perform the following calculation to the right number of sig figs:
[(1.7 × 106) ÷ (2.63 × 105)] + 6.96
2953views28rank8comments - Open Question
Express the product of 2.2 mm and 5.00 mm using the correct number of significant digits.
585views - Open Question
What answer should be reported with the correct number of significant figures
626views - Open Question
Joseph divides 8.64 by 2.0. How many significant figures should his answer have?
533views - Open Question
Zachary adds 26.45 g to 2.55 g. How many significant figures should his answer have?
392views - Multiple ChoiceSolve the following, considering significant figures: 2.3 + 4.94 + 3 =440views
- Multiple ChoiceWhich of the following is the correct rule for determining the number of significant figures in the result of an addition or subtraction operation?35views