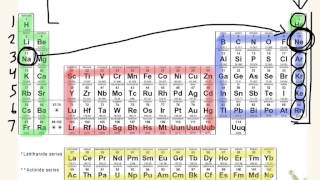
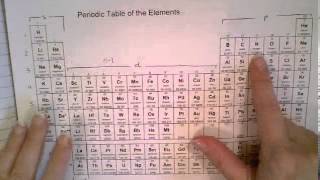
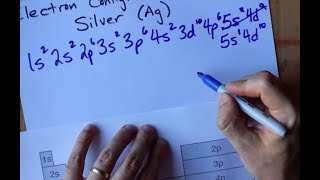10. Periodic Properties of the Elements
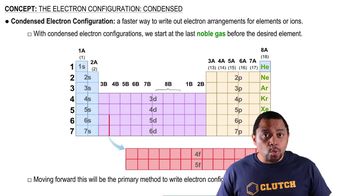
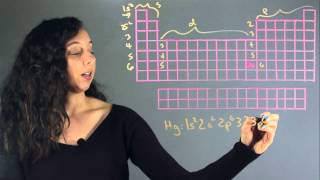
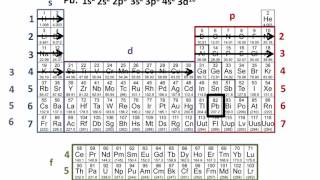
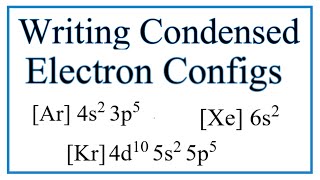
The Electron Configuration: Condensed
10. Periodic Properties of the Elements
The Electron Configuration: Condensed
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
Write the condensed electron configuration and electron orbital diagram for the following element: Zinc
1718views25rank3comments - Multiple ChoiceWhich electron configuration violates the Pauli exclusion principle?962views
- Open Question
Which is the electron configuration for nobelium (No)? [Rn]7s25f14 [Rn]7s25f7 [Ne]3s23p7 [Xe]6s25d1
976views - Open Question
Give the ground-state electron configuration for silicon ( Si ) using noble-gas shorthand.
567views - Open Question
Identify the atom with the ground-state electron configuration shown for its valence shell. 4𝑠23𝑑10
562views - Open Question
Using core notation, what is the abbreviated electron configuration of yttrium (Y)?
623views - Multiple ChoiceWhich of the following condensed electron configurations represents a noble gas in its ground state?29views
- Multiple ChoiceWhich of the following is the correct condensed electron configuration for selenium (Z = 34)?28views