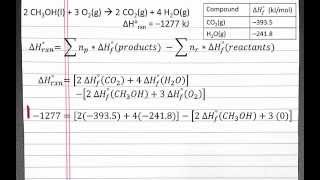
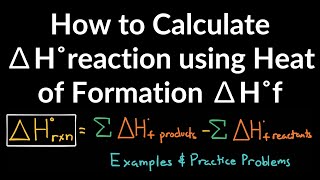
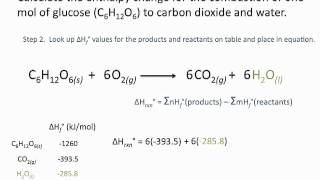8. Thermochemistry
Formation Equations
8. Thermochemistry
Formation Equations
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Which of the following represents the formation equation for barium nitrate, Ba(NO3)2?
1165views7rank - Multiple Choice
Identify a substance that is not in its standard state.
1979views - Multiple Choice
Ibuprofen is used as an anti-inflammatory agent used to deal with pain and bring down fevers. If it has a molecular formula of C13H18O2, determine the balanced chemical equation that would give you directly the enthalpy of formation for ibuprofen.
1358views6rank - Open Question
Which substance has ΔHf defined as 0 kJ/mol? H2O(s), Ne(l), F2(g), CO2(g).
981views - Open Question
How many kilojoules of heat will be released when exactly 1 mole of manganese, Mn, is burned to form Mn3O4(s) at standard state conditions?
890views - Open Question
Using Hess's law, calculate the heat of formation for Cao(s) using the following reaction.
781views - Multiple ChoiceWhich of the following is the correct chemical equation for the formation of BF3(g) from its elements in their standard states?558views