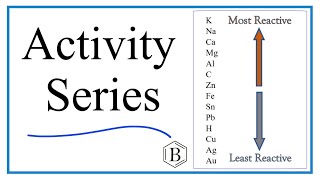
6. Chemical Quantities & Aqueous Reactions
Activity Series
6. Chemical Quantities & Aqueous Reactions
Activity Series
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
Based on your understanding of activities determine if a reaction occurs and if so provide the products formed.
Ba (s) + H2O (g) →
792views1rank2comments - Multiple Choice
Based on your understanding of activities determine if a reaction occurs and if so provide the products formed.
Zn (s) + NiCl2 (aq) →
713views5rank - Multiple Choice
If the activity of halogens is stated as:Fluorine > Chlorine > Bromine > Iodine, determine if a reaction occurs and if so provide the products formed.
Cl2 (g) + AlBr3 (aq) →
768views7rank1comments - Open QuestionNickel metal is added to a solution of copper(ii) nitrate.1067views
- Open QuestionChromium metal is immersed in an aqueous solution of cobalt(ii) chloride.1266views
- Open Question
What is produced during the replacement reaction of Cu(NO3)2 and Zn?
567views - Open Question
What will happen when a less reactive metal is added to a metal salt solution?
564views - Multiple ChoiceWhich of the following metals will react with a solution of copper(II) sulfate, based on the activity series?381views