2. Atoms & Elements
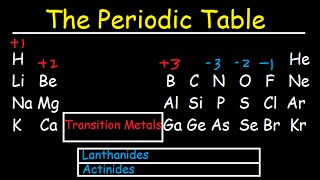
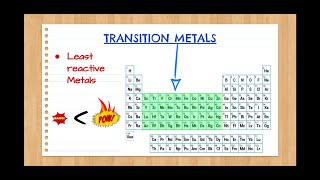
Periodic Table: Classifications
2. Atoms & Elements
Periodic Table: Classifications
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Open Question
For metalloids on the periodic table, how do the group number and the period number relate?
684views - Open Question
Which of these properties is the best one to use for the identification of an element?
511views - Open QuestionClassify each element as a metal, nonmetal, or semimetal.912views
- Open Question
Of the elements Pt, V, Li, and Kr, which is a nonmetal?
736views - Multiple ChoiceHow many naturally occurring elements are there on the periodic table?258views
- Multiple ChoiceHow many elements occur naturally on the periodic table?469views
- Multiple ChoiceThe periodic table provides which of the following information regarding an element?295views
- Multiple ChoiceIn the classification of matter, what is the broadest category and what is the most specific category?486views