9. Quantum Mechanics
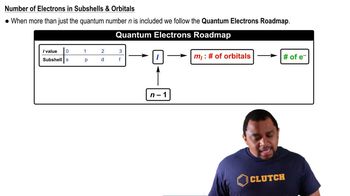
Quantum Numbers: Number of Electrons
9. Quantum Mechanics
Quantum Numbers: Number of Electrons
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Determine the number of electrons that can have the following set of quantum numbers:n = 3, ml = 0.
1130views4rank1comments - Multiple Choice
Determine the number of electrons that can have the following set of quantum numbers:n = 2, ms = –1/2.
1077views2rank - Multiple Choice
Determine the number of electrons that can have the following set of quantum numbers.
n = 4, l = 3, ml = – 1
1112views6rank1comments - Multiple Choice
Determine the number of electrons that can have the following set of quantum numbers.
n = 4, mL = – 1, ms = –1/2
1100views5rank2comments - Open QuestionHow many electrons in an atom could have these sets of quantum numbers?580views
- Open QuestionWhat is the maximum number of electrons in an atom that can have the following quantum numbers?475views
- Open QuestionWhat is the maximum number of electrons that can occupy each of the following subshells?494views
- Open Question
What is the maximum number of electrons that can exist in the g sublevel?
511views