19. Chemical Thermodynamics
Entropy
19. Chemical Thermodynamics
Entropy
Showing 9 of 9 videos
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
An ideal gas is allowed to expand at constant temperature. What are the signs of ∆H, ∆S & ∆G.
2182views2rank7comments - Multiple Choice
Consider the freezing of liquid water at 30°C. For this process what are the signs for ∆H, ∆S, and ∆G?
2616views1rank4comments - Multiple Choice
Predict the sign of ∆S in the system for each of the following processes:
a) Ag+ (aq) + Br - (aq) → AgBr (s)
b) CI2 (g) → 2 CI - (g)
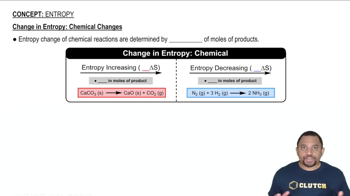
c) CaCO3 (s) → CaO (s) + CO2 (g)
d) Pb (s) at 50°C → Pb (s) at 70°C
2977views4rank3comments - Multiple Choice
For each of the following reactions state the signs of ∆H (enthalpy) and ∆S (entropy):
a) Fusion of ice.
b) Sublimation of CO2
c) Vaporization of aqueous water.
d) Deposition of chlorine gas.
e) Condensation of water vapor.
2498views2rank3comments - Open QuestionWithout doing any calculations, determine the sign of δssys for each of the following chemical reactions.753views
- Open QuestionWhich of the following liquid substances has the highest vapor pressure at its normal boiling point?787views
- Open QuestionRank these systems in order of decreasing entropy. rank from highest to lowest entropy. to rank items as equivalent, overlap them.1599views2rank1comments
- Open QuestionWhich of the following is a measure of randomness in a system?900views