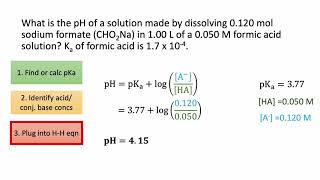
18. Aqueous Equilibrium
Intro to Buffers
18. Aqueous Equilibrium
Intro to Buffers
Showing 7 of 7 videos
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Multiple Choice
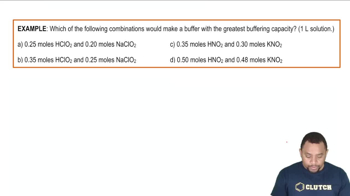
Which one of the following combinations does not create a buffer?
2839views5rank11comments - Multiple Choice
Which of the following combinations can result in the formation of a buffer?
2842views5rank7comments - Multiple Choice
Which of the following combinations can result in the formation of a buffer?
2759views1rank5comments - Multiple Choice
A buffer solution is comprised of 50.0 mL of a 0.100 M HC2H3O2 and 60.0 mL of a 0.100 M NaC2H3O2. Which of the following actions would completely destroy the buffer?
4063views3rank9comments - Open Question
A buffer resists change in ph when ___________ amount of acid or base is added to it.
591views - Open QuestionWhich of the following solutions represents a good buffer system?492views
- Open Question
A solution that resists a change in ph when an acid or base is added to it is a(n) __________.
452views - Open Question
Select the statement that best describes a buffer. see concept 3.3 (page)
482views