2. Atoms & Elements
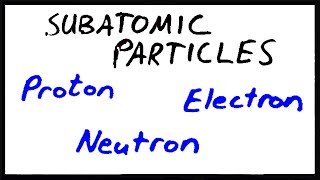
Subatomic Particles
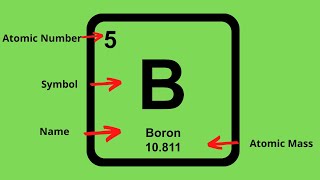
2. Atoms & Elements
Subatomic Particles
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
According to the table above, how many electrons are required to produce a charge of –2.0 C?
1961views7rank2comments - Multiple Choice
If the charge and mass of one proton is 1.60218 x 10-19 C and 1.673 x 10-24 g respectively, what is the charge of 378 kg of protons?
1792views13rank - Multiple ChoiceWhich of the following statements is true?1016views
- Multiple ChoiceWhich of the following has the largest number of neutrons?1039views
- Open Question
Rounded to the nearest whole number, how many protons are in an atom of krypton?
719views - Open Question
Oxygen has an atomic number of 8. What can you conclude from this fact?
936views - Open Question
On the periodic table, gold has the atomic number 79. This means that
1459views - Open Question
How many neutrons are in an atom of copper? Round your answer to the nearest whole number.
790views