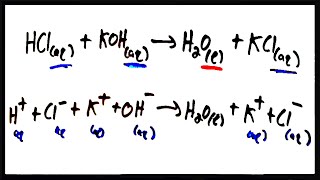6. Chemical Quantities & Aqueous Reactions
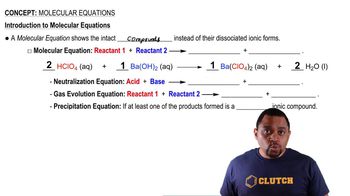
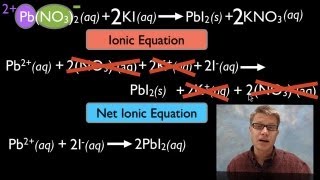
Molecular Equations
6. Chemical Quantities & Aqueous Reactions
Molecular Equations
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Predict whether a chemical reaction occurs and write the balanced molecular equation.
Ag2SO4 (aq) + KCl (aq) →
2089views17rank4comments - Multiple Choice
Predict whether a chemical reaction occurs and write the balanced molecular equation.
MgBr2 (aq) + NaC2H3O2 (aq) →
1844views9rank4comments - Multiple Choice
Determine the balanced equation for the neutralization equation
Ca(OH)2 (aq) + HCN (aq) →
2098views11rank4comments - Multiple ChoiceWhich of the following compounds is insoluble in water?1443views
- Open Question
Consider the reaction 2 Al(OH)3 + 3 H2SO4 → x + 6y. What are x and y?
900views - Open Question
An acid (x) reacts with a base (y) to produce Mg3(PO4)2. What are x and y?
714views - Open Question
Which type of chemical reaction occurs in C6H12 + 9O2 right arrow 6CO2 + 6H2O?
561views - Open Question
What is produced during the replacement reaction of Ba(NO3)2 and Na2SO4?
615views