10. Periodic Properties of the Elements
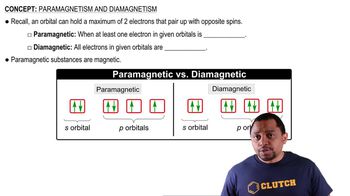
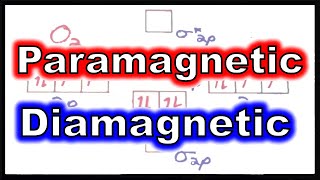
Paramagnetism and Diamagnetism
10. Periodic Properties of the Elements
Paramagnetism and Diamagnetism
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Which of the following atoms has the most unpaired electrons?
2198views8rank - Multiple Choice
Write the condensed electron configuration for the nickel (III) ion and state if it is paramagnetic or diamagnetic.
1585views9rank1comments - Multiple Choice
Write the condensed electron configuration for the copper (I) ion and is it magnetic?
1195views7rank1comments - Multiple ChoiceWhich one of the following atoms or ions is diamagnetic?889views
- Open Question
In the ground-state electron configuration of Fe3+, how many unpaired electrons are present?
707views - Open QuestionSelect the element(s) that will have one unpaired electron in the p orbital.1007views
- Open QuestionIdentify the general outer electron configuration for each group of elements shown in this periodic table outline.733views
- Open QuestionSort the following atom or ions as paramagnetic or diamagnetic according to the electron configurations determined in part a.568views