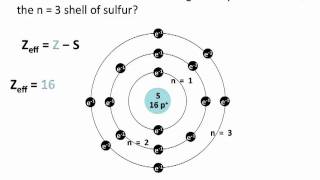
10. Periodic Properties of the Elements
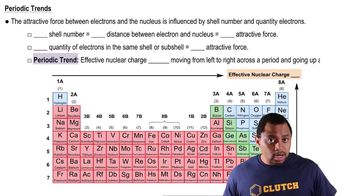
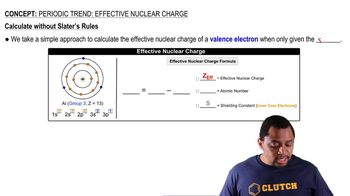
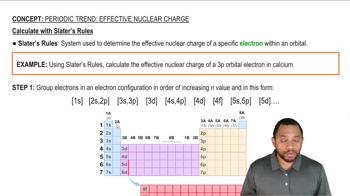
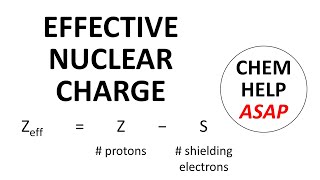
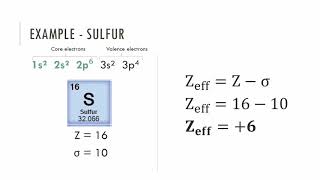
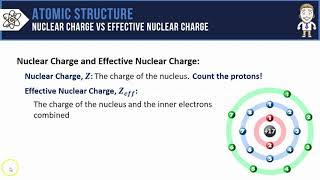
Periodic Trend: Effective Nuclear Charge
10. Periodic Properties of the Elements
Periodic Trend: Effective Nuclear Charge
Showing 6 of 6 videos
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
What is the identity of an element when the effective nuclear charge of its valence electrons is 18 while its shielding constant is 5?
1534views6rank1comments - Multiple Choice
In which orbital does an electron in a sulfur atom experience the greatest shielding?
1877views2rank - Multiple Choice
Using Slater's Rules calculate the effective nuclear charge of the 4d orbital electron in iodine.
2963views2comments - Open Question
The shielding of electrons gives rise to an effective nuclear charge, Zeff, which explains why boron is larger than oxygen. Estimate the approximate Zeff felt by a valence electron of boron and oxygen, respectively?
663views - Open Question
Calculate Zeff for a 3d electron in a copper atom, Cu.
1122views - Open QuestionClassify each statement about effective nuclear charge, 𝑍eff , as true or false.858views
- Open QuestionRank the elements by effective nuclear charge, 𝑍eff, for a valence electron.797views
- Multiple Choice
Rank the following elements by effective nuclear charge, ZEff, for a valence electron: Kr, Se, Ca, K, Ge
763views