13. Liquids, Solids & Intermolecular Forces
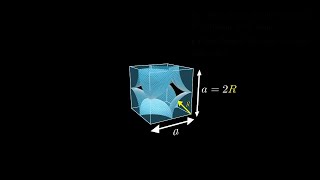
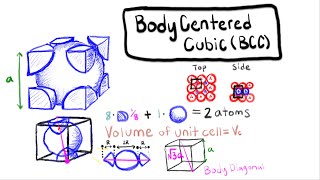
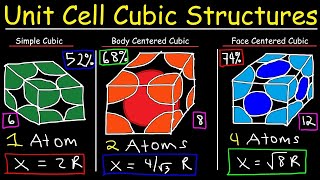
Simple Cubic Unit Cell
13. Liquids, Solids & Intermolecular Forces
Simple Cubic Unit Cell
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
Polonium crystallizes with a primitive cubic structure. It has a density of 9.4 g/cm3, a radius of 167 pm, and a molar mass of 209 g/mol. Calculate the number of atoms in one mole of Polonium.
1619views5rank1comments - Open Question
How many atoms are in a simple cubic (primitive cubic) unit cell?
601views - Open Question
How many total (or composite) atoms are contained in a unit cell of primitive cubic arrangement?
657views - Multiple ChoiceGallium crystallizes in a primitive cubic unit cell. The length of an edge of this cube is 362 pm. What is the radius of a gallium atom?367views
- Multiple ChoicePolonium metal crystallizes in a simple cubic arrangement. If the unit cell edge length is 334 pm, what is the atomic radius of polonium?409views
- Multiple ChoiceHow many atoms are present in a single simple cubic unit cell?57views