8. Thermochemistry
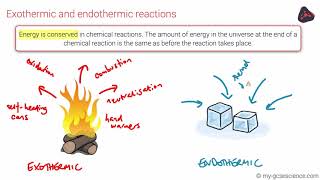
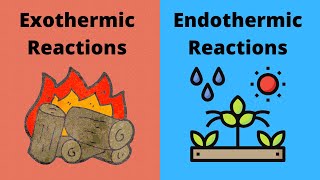
Endothermic & Exothermic Reactions
8. Thermochemistry
Endothermic & Exothermic Reactions
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Multiple ChoiceWhich of the following exchanges of energy has a positive sign for heat (relative to the system)?771views
- Multiple ChoiceIf a cylinder equipped with a piston expands against an external pressure of 1.25 atm, increasing the volume of a cylinder by 0.500 L and releasing 25.0 J of heat, what is ΔE for the process?920views
- Multiple ChoiceA 0.1250-g sample of octane (C8H18) is burned in a bomb calorimeter with a heat capacity of 5.1 × 102 J/℃. The temperature inside the calorimeter increases by 11.8℃. What is the ΔE (in kJ/mol octane) for the combustion of octane?1028views
- Multiple ChoiceWhich of the following is the result of an exothermic process?830views
- Open Question
The following reaction A --> B + C + heat is a(n) _____ reaction.
716views - Open Question
The enthalpy of formation of water is -285.8 kJ/mol. What can be inferred from this statement?
772views - Open QuestionWhich of the following processes is endothermic?899views
- Open QuestionWhich one of the following is an endothermic process?789views