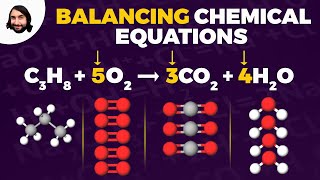
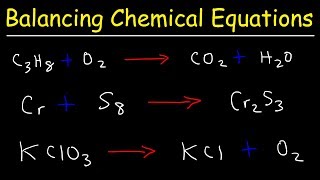
3. Chemical Reactions
Balancing Chemical Equations
3. Chemical Reactions
Balancing Chemical Equations
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Open Question
Determine the total sum of the coefficients after balancing the following equation.
___ C2H5SH (g) + ___ O2 (g) → ___ CO2 (g) + ___ H2O (l) + ___ SO2 (g)
2661views44rank6comments - Multiple ChoiceBalancing a chemical equation1406views
- Multiple ChoiceWhen the following equation is balanced, what is the coefficient in front of H2O?
___ Ba(OH)2 + ___ H3PO4 → ___ H2O + ___ Ba3(PO4)2
996views - Multiple ChoiceIn the following equation what is the mole ratio of NaOH to H2O, respectively?
___NaOH (aq) + ___ H3PO4(aq) → ___ H2O(l) + ___ Na3PO4(aq)
888views - Open Question
Write the balanced equation for the following by inserting the correct coefficients in the blanks.
___ Al4C3 (s) + ___ H2O (l) → ___ Al2O3 (s) + ___ CH4 (g)
1153views35rank2comments - Multiple Choice
Determine the balanced chemical equation when ethanol, C2H6O is ignited in the presence of air.
1095views16rank2comments - Open Question
For each molecule of C4H8 that reacts, how many molecules of carbon dioxide and water are produced?
675views - Open Question
H3PO4 + Mg(OH)2 → Mg3(PO4)2 + H2O
1745views