6. Chemical Quantities & Aqueous Reactions
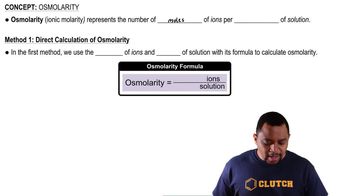
Osmolarity
6. Chemical Quantities & Aqueous Reactions
Osmolarity
Showing 5 of 5 videos
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
Which of the following solutions will have the highest concentration of bromide ions?
2599views12rank - Multiple Choice
How many milligrams of nitride ions are required to prepare 820 mL of 0.330 M Ba3N2 solution?
1739views8rank - Multiple Choice
How many bromide ions are present in 65.5 mL of 0.210 M GaBr3 solution?
1692views9rank1comments - Open Question
What is the concentration of K+ in 0.15 M of K2S?
721views - Open Question
What is the concentration of K+ in 0.15 m of K2S?
869views - Open Question
What is the molar concentration of sodium ions in a 0.350 m Na3PO4 solution?
667views - Open QuestionWhat is the molarity of cl− in each solution?864views
- Multiple Choice0.0201 g of aspirin is dissolved in enough water to produce a 100.0 mL solution. The osmotic pressure of this solution is 0.0271 atm at 301 K. Aspirin has a van't Hoff factor of 1. Determine the molar mass of aspirin.297views