1. Intro to General Chemistry
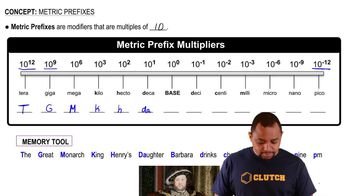
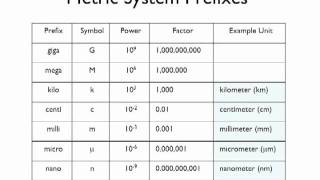
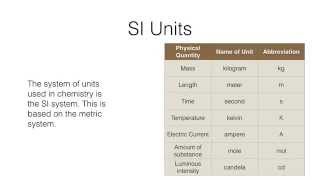
Metric Prefixes
1. Intro to General Chemistry
Metric Prefixes
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Which quantity in the following pair is smaller?
3056views52rank4comments - Open Question
Use the prefix multipliers to express each measurement without any exponents.
a) 32 x 10-13 L
b) 7.3 x 106 g
c) 18.5 x 1011 s
4166views57rank6comments - Open Question
Use scientific notation to express each quantity with only the base unit.
a) 83 µm
b) 193 kg
c) 2.7 mmol
2297views46rank13comments - Multiple Choice
If a room has a volume of 1.15 x 108 cm3, what is the volume in km3?
2967views66rank9comments - Open QuestionComplete each row of the table below by filling in the missing prefix or missing exponent.1293views
- Open Question
The metric equivalent of 0.001 gram is __________.
585views - Multiple ChoiceWhich of the following metric prefixes corresponds to 10^6?57views
- Multiple ChoiceHow many grams are present in 1000 milligrams?84views