10. Periodic Properties of the Elements
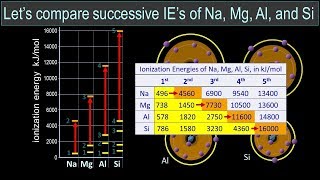
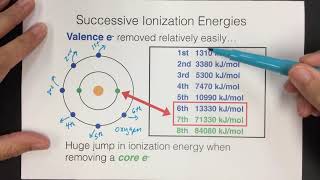
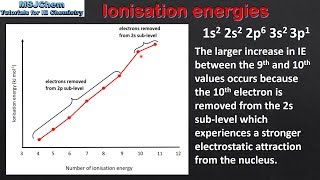
Periodic Trend: Successive Ionization Energies
10. Periodic Properties of the Elements
Periodic Trend: Successive Ionization Energies
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Which of the following represents the third ionization of Mn?
1151views4rank - Multiple Choice
Of the following atoms, which has the largest third ionization energy?
2203views5rank4comments - Open QuestionExamine the following set of ionization energy values for a certain element. how many valence electrons does an atom of the neutral element possess?687views
- Open QuestionWhich ionization process requires the most energy?1136views
- Open QuestionFor each of the following elements, predict where the "jump" occurs for successive ionization energies (ie).543views
- Open QuestionWhich ionization process requires the most energy643views
- Multiple Choice
The successive ionization energies for an unknown element are:
IE1 = 896 kJ/molIE2 = 1752 kJ/molIE3 = 14,807 kJ/molIE4 = 17,948 kJ/mol
To which family in the periodic table does the unknown element most likely belong?
613views2rank - Multiple ChoiceWhich period 3 element has the following consecutive ionization energies: Ei1 = 578 kJ/mol, Ei2 = 1820 kJ/mol, Ei3 = 2750 kJ/mol, Ei4 = 11,600 kJ/mol?254views