3. Chemical Reactions
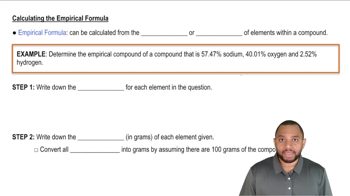
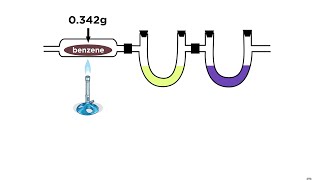
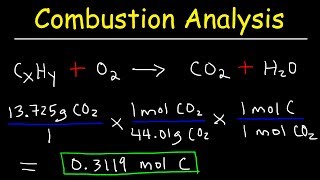
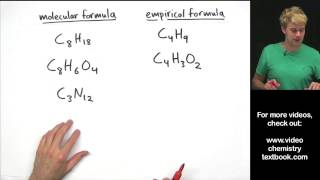
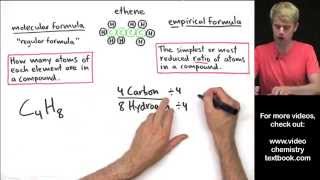
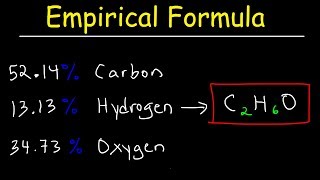
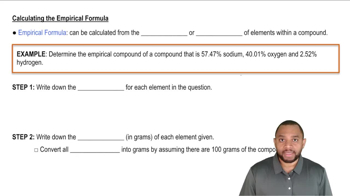
Empirical Formula
3. Chemical Reactions
Empirical Formula
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Multiple ChoiceWhich of the following could be both a molecular formula and an empirical formula?1115views
- Multiple ChoiceCovalent compounds1027views1rank
- Multiple ChoiceIn order to make 1 s’ more, you need 2 graham crackers, 1 piece of chocolate, and 1 marshmallow. How many s’mores can be made from the following ingredients?
7 graham crackers, 6 pieces of chocolate, and 5 marshmallows
995views1rank - Multiple Choice
A chemist wishing to identify a compound determines the masses of its elements as: 1.445 g S and 6.391 g Cl. Determine its empirical formula.
995views21rank - Open Question
What is the empirical formula of a compound that contains only iron and oxygen and is 22.27% oxygen?
882views - Open Question
In general, what does a subscript (such as the "2" in H2) tell you about the molecule?
929views - Open Question
How many atoms of oxygen are in the chemical formula 2Ca(ClO2)2?
895views - Open Question
Some of the formulas below could be either molecular or empirical formulas; however, some could only be molecular formulas. Which of the following formulas must be molecular formulas? Select all that apply.
616views