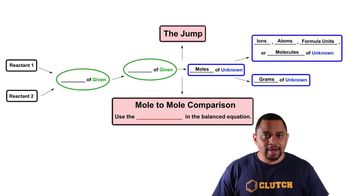
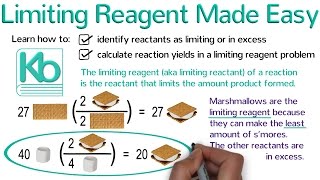
3. Chemical Reactions
Limiting Reagent
Learn with other creators
Practice this topic
- Multiple ChoiceWhen the amounts of all reactants are given for a chemical equation, the amount that is used to calculate the amount of product formed is the675views1rank
- Multiple Choice
The following reaction shows the mineral ilmenite, FeTiO3, being reacted with chlorine gas and sand in order to extract titanium (IV) chloride.
FeTiO3 (s) + 3 Cl2 (g) + 3 C (s) → 3 CO (g) + FeCl2 (s) + TiCl4 (g)
Assuming a 100% yield, how many grams of titanium (IV) chloride can be extracted when reacting 18.0 g ilmenite, 30.0 g Cl2 and 40.0 g C?
895views7rank1comments - Multiple Choice
Lithium solid reacts with oxygen gas to create lithium oxide solid.
4 Li (s) + O2 (g) → 2 Li2O (s)
If 131 g of Li are allowed to react with 215 g O2, how many kilograms of the excess reactant would remain?
1356views10rank3comments - Open Question
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia. N2(g) + 3H2(g) ⟶ 2NH3(g) there are four molecules of nitrogen and nine molecules of hydrogen present in the diagram. When the reaction is complete, how many molecules of NH3 are produced?
961views - Open Question
1 moles of mn reacts with 1 moles of O2. What is the theoretical yield of the product? 𝗠𝗻(𝘀) + 𝗢2(𝗴) → 𝗠𝗻𝗢2(𝘀)
646views - Open QuestionHow many co2 molecules would be formed from the reaction mixture that produces the greatest amount of products?1875views
- Open QuestionAfter the reaction, how much octane is left?858views
- Multiple ChoiceIf you have 24 grams of magnesium (Mg) and 16 grams of oxygen (O₂), which is the limiting reactant in the reaction 2Mg + O₂ → 2MgO?485views