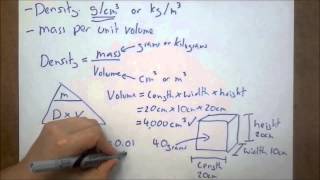
1. Intro to General Chemistry
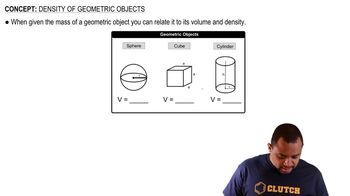
Density of Geometric Objects
1. Intro to General Chemistry
Density of Geometric Objects
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
A copper wire (density = 8.96 g/cm3) has a diameter of 0.32 mm. If a sample of this copper wire has a mass of 21.7 g, how long is the wire?
4069views6rank2comments - Multiple Choice
If the density of a certain spherical atomic nucleus is 1.0 x 1014 g/cm3 and its mass is 3.5 x 10-23 g, what is the radius in angstroms? (Å= 10−10 m)
1970views6rank2comments - Multiple ChoiceThe two objects shown below have the same mass. Which has the largest density?937views
- Multiple ChoiceA block of metal has a width of 3.2 cm, a length of 17.1 cm, and a height of 4.3 cm. Its mass is 1.1 kg. Calculate the density of the metal in g/cm³.246views