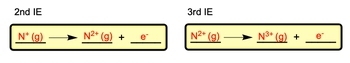
The first ionization energy, denoted as \( I_{e1} \), refers to the energy required to remove the first electron from a gaseous atom, resulting in a positively charged ion. For nitrogen, this energy is approximately 1402 kJ/mol. When an electron is removed, the atom transitions from a neutral state to a positively charged state, specifically \( N^+ \) in this case.
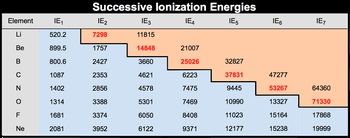
Successive ionizations involve the removal of additional electrons one at a time, rather than all at once. For instance, after removing the first electron, nitrogen becomes \( N^+ \). The second electron can then be removed, leading to the formation of \( N^{2+} \). This process continues, with each removal increasing the positive charge of the ion. Thus, the second ionization energy, \( I_{e2} \), is the energy needed to remove the second electron from \( N^+ \), and the third ionization energy, \( I_{e3} \), is the energy required to remove the third electron from \( N^{2+} \).
In summary, the ionization process for nitrogen can be represented as follows:
- First ionization: \( N \rightarrow N^+ + e^- \) (with \( I_{e1} = 1402 \, \text{kJ/mol} \))
- Second ionization: \( N^+ \rightarrow N^{2+} + e^- \) (with \( I_{e2} \))
- Third ionization: \( N^{2+} \rightarrow N^{3+} + e^- \) (with \( I_{e3} \))
Each successive ionization requires more energy due to the increased positive charge of the ion, which leads to a stronger attraction between the remaining electrons and the nucleus.