4. Biomolecules
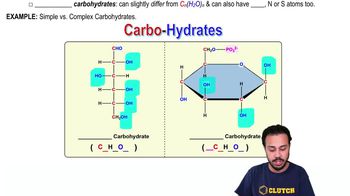
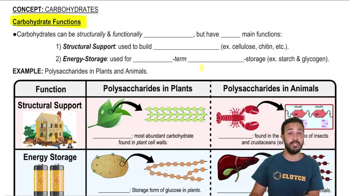
Carbohydrates
4. Biomolecules
Carbohydrates
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Multiple Choice
Which of the following chemical formulas represents that of a simple carbohydrate?
6000views103rank - Multiple Choice
Monosaccharides are linked together via a ______________ reaction, forming a _____________bond.
6012views71rank - Multiple Choice
Which of the following chemical reactions results in energy release when glycosidic bonds are broken?
5634views69rank - Multiple Choice
Animal cells store energy in the form of _________, and plant cells store energy in the form of ___________.
6664views62rank