8. Respiration
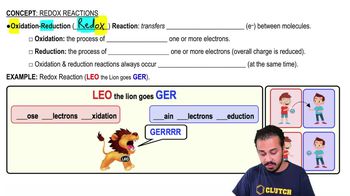
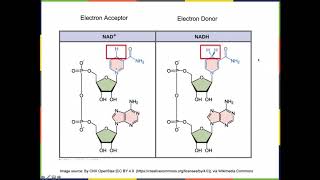
Redox Reactions
8. Respiration
Redox Reactions
Additional 11 creators.
Learn with other creators
Showing 14 of 14 videos
Practice this topic
- Multiple Choice
Oxidation is the _________________________, and reduction is the _________________________.
6317views76rank - Multiple Choice
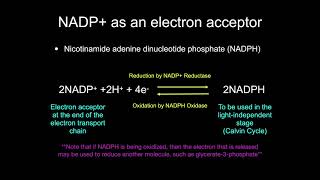
When glucose donates electrons to NAD+ creating NADH, the glucose molecule becomes:
6055views60rank - Multiple Choice
An electron carrier before it harvests energy from glucose molecules in a series of gradual steps is:
5570views93rank - Multiple ChoiceA molecule becomes more oxidized when it __________.2508views