1. Intro to General Chemistry
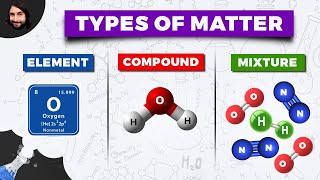
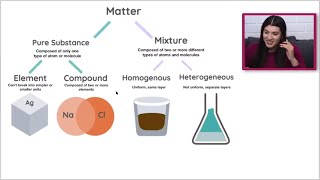
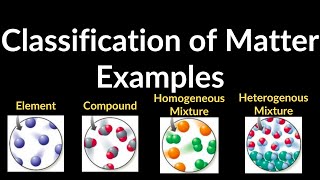
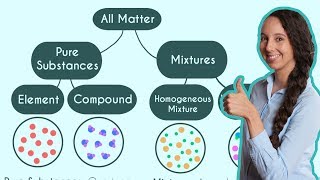
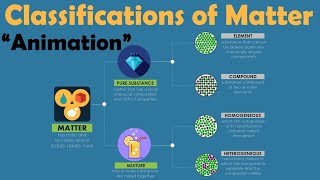
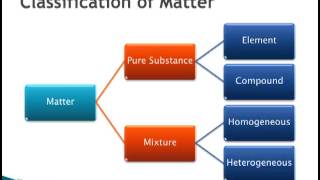
Classification of Matter
1. Intro to General Chemistry
Classification of Matter
Additional 10 creators.
Learn with other creators
Showing 13 of 13 videos
Practice this topic
- Multiple Choice
Which of the following statements is true?
13885views193rank13comments - Multiple Choice
Choose the homogeneous mixture from the list below.
10794views118rank9comments - Open Question
Classify each of the following as an element, compound or mixture.
a) Ammonia, NH3
b) Gold bar
c) Orange juice
d) Wine
e) Saline solution
6914views130rank6comments - Multiple ChoiceConsider the following data obtained by decomposing samples of carbon dioxide, CO2, into its component elements.
Mass of CO2 and Components Sample Mass CO2 Mass C Mass O2 1 44.00 g 12.00 g 32.00 g 2 22.00 g 6.00 g 16.00 g 3 88.00 g 24.00 g 64.00 g
Which of the following statements is consistent with this data?1861views2rank - Multiple ChoiceAtoms __________ .1557views1rank
- Open Question
A _____ is a physical combination of matter in any proportions.
1763views3rank - Open Question
Based on the given information, what is the best classification for this sample of crude oil?
1366views - Open Question
What is the basic form of matter that cannot be broken down any further?
1487views