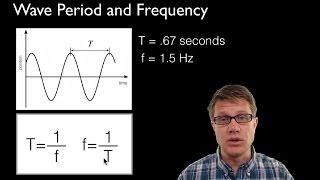
9. Quantum Mechanics
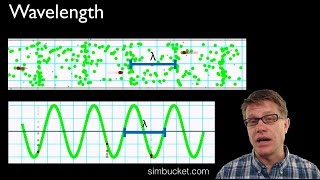
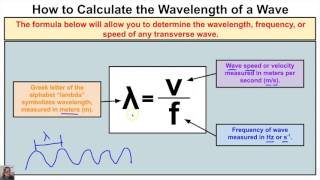
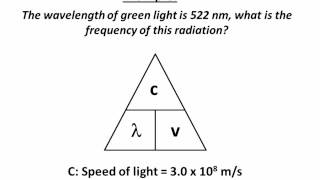
Wavelength and Frequency
9. Quantum Mechanics
Wavelength and Frequency
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
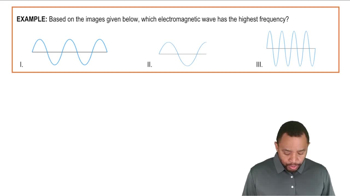
Which energy wave would have the highest frequency from the wavelengths provided?
2744views53rank1comments - Multiple ChoiceWhich of the following statements is/are true concerning electrons?780views
- Multiple ChoiceAt what speed must a 145-g baseball travel to have a wavelength of 1.15 × 10−34 m?759views
- Open QuestionConsider the two waves shown here (figure 1), which we will consider to represent two electromagnetic radiations.1014views
- Multiple ChoiceA C–C bond has an average bond strength of 350 kJ/mol. What is the longest wavelength (lowest energy) light, in nm, that can break the average C–C bond? (1 m = 10⁹ nm)426views