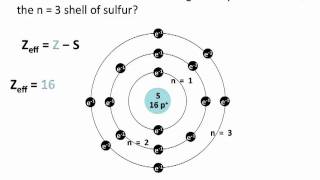
10. Periodic Properties of the Elements
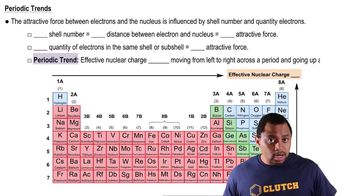
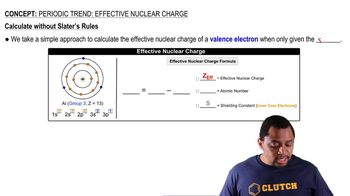
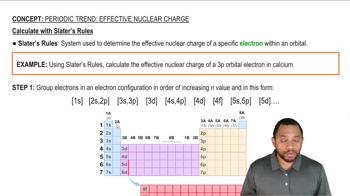
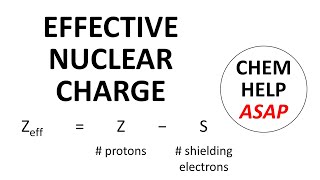
Periodic Trend: Effective Nuclear Charge
Problem 60
Textbook Question
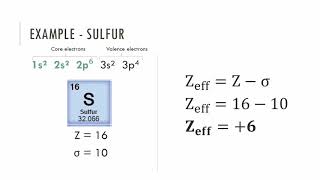
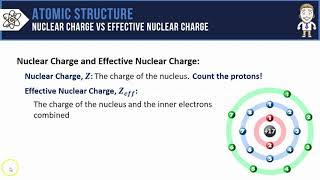
Textbook QuestionIn Section 3.6, we estimated the effective nuclear charge on beryllium's valence electrons to be slightly greater than 2+. What would a similar treatment predict for the effective nuclear charge on boron's valence electrons? Would you expect the effective nuclear charge to be different for boron's 2s electrons compared to its 2p electron? In what way? (Hint: Consider the shape of the 2p orbital compared to that of the 2s orbital.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
974
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos