8. Thermochemistry
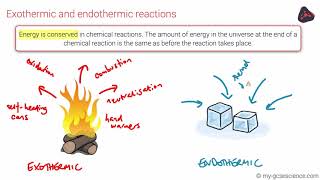
Endothermic & Exothermic Reactions
Problem 48b
Textbook Question
Textbook QuestionConsider the decomposition of liquid benzene, C6H61l2, to gaseous acetylene, C2H21g2: C6H61l2 ¡ 3 C2H21g2 H = +630 kJ (c) Which is more likely to be thermodynamically favored, the forward reaction or the reverse reaction?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
264
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos