1. Intro to General Chemistry
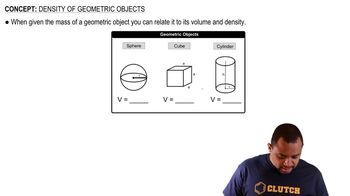
Density of Geometric Objects
Problem 117
Textbook Question
Textbook QuestionBrass is a copper–zinc alloy. What is the mass in grams of a brass cylinder having a length of 1.62 in. and a diameter of 0.514 in. if the composition of the brass is 67.0% copper and 33.0% zinc by mass? The density of copper is 8.92 g/cm3, and the density of zinc is 7.14 g/cm3. Assume that the den-sity of the brass varies linearly with composition.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1250
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos