1. Intro to General Chemistry
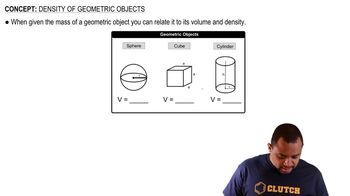
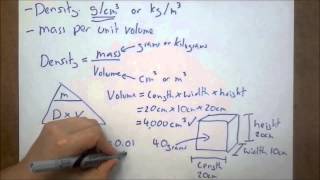
Density of Geometric Objects
Problem 91b
Textbook Question
Textbook QuestionVery small semiconductor crystals, composed of approximately 1000 to 10,000 atoms, are called quantum dots. Quantum dots made of the semiconductor CdSe are now being used in electronic reader and tablet displays because they emit light efficiently and in multiple colors, depending on dot size. The density of CdSe is 5.82 g/cm3. (c) What is the mass of one 6.5-nm CdSe quantum dot?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
305
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos