1. Intro to General Chemistry
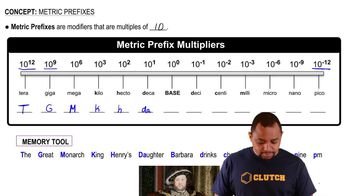
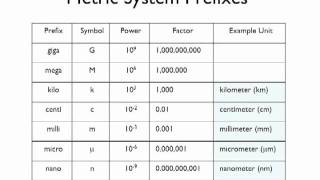
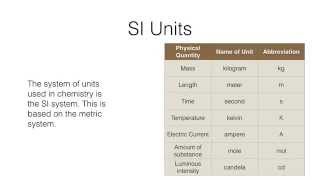
Metric Prefixes
Problem 15
Textbook Question
Textbook QuestionAerogels are transparent, low-density materials that are nearly 99.8% empty space and excellent insulators against hot and cold. The density of a silica-based aerogel is 3.0 mg/cm3. What is the density in units of g/m3? (LO 1.17) (a) 3.0 * 10-3 g/m3 (b) 3.0 * 101 g/m3 (c) 3.0 g/m3 (d) 3.0 * 103 g/m3
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
575
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos