3. Chemical Reactions
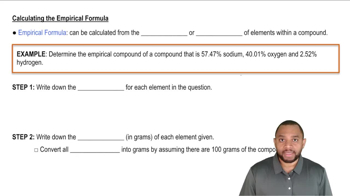
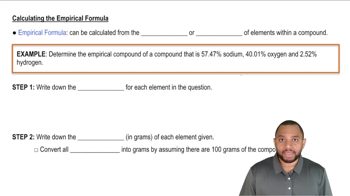
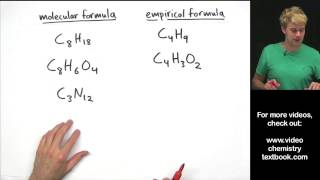
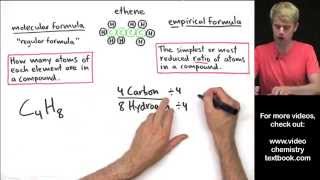
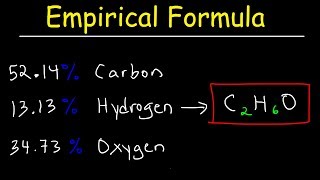
Empirical Formula
Problem 94
Textbook Question
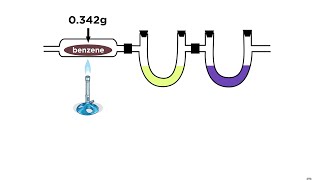
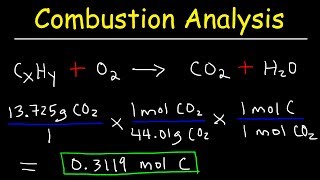
Textbook QuestionA 45.2-mg sample of phosphorus reacts with selenium to form 131.6 mg of the selenide. Determine the empirical formula of phosphorus selenide.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1821
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos