6. Chemical Quantities & Aqueous Reactions
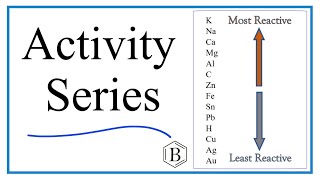
Activity Series

Get help from an AI Tutor
Ask a question to get started.
Problem 124
Textbook Question
Textbook QuestionChlorine dioxide gas 1ClO22 is used as a commercial bleaching agent. It bleaches materials by oxidizing them. In the course of these reactions, the ClO2 is itself reduced. (b) Why do you think that ClO2 is reduced so readily?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
407
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos