7. Gases
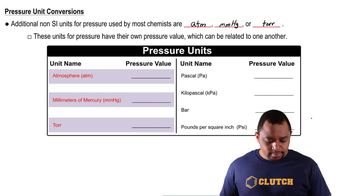
Pressure Units
7. Gases
Pressure Units
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Open Question
A set of bookshelves rests on a hard floor surface on four legs, each having a cross-sectional dimension of 3.0 x 4.1 in contact with the floor. The total mass of the shelves plus the books stacked on them is 266 kg.
Calculate the pressure in pascals exerted by the shelf footings on the surface.
311views1rank - Open Question
What is the pressure (in atmospheres) of the gas inside the container connected to an open-end, mercury-filled manometer as shown in the picture? (figure 1) the atmospheric pressure is 0.95 atm.
869views - Open Question
If you measure pressure in bars instead of atmospheres, calculate the corresponding value of r in l−bar/mol−k.
706views - Open QuestionForce per unit area is termed ________.1216views
- Multiple ChoiceA 5.00-L tank contains helium gas at 1.50 atm. What is the pressure of the gas in mmHg?582views
- Multiple ChoiceA gas at a pressure of 325 torr exerts a force of ________ N on an area of 5.5 m². What is the force exerted by the gas?456views
- Multiple ChoiceAt a pressure of 937 mbar, what would be the corresponding height of the mercury in the column of a mercury barometer in millimeters of mercury (mmHg)?569views
- Multiple ChoiceGiven a barometric pressure of 762.4 mmHg, what is the pressure in atmospheres (atm)?540views