3. Chemical Reactions
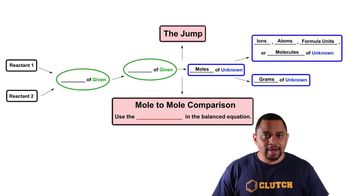
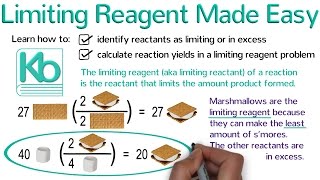
Limiting Reagent
Problem 78a
Textbook Question
Textbook QuestionAluminum hydroxide reacts with sulfuric acid as follows: 2 Al1OH231s2 + 3 H2SO41aq2¡Al21SO4231aq2 + 6 H2O1l2 Which is the limiting reactant when 0.500 mol Al1OH23 and 0.500 mol H2SO4 are allowed to react?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
1021
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos