3. Chemical Reactions
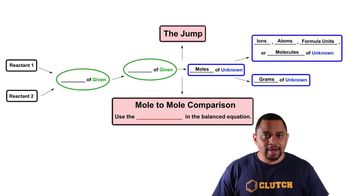
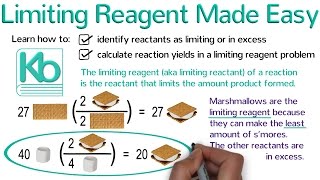
Limiting Reagent
Problem 84
Textbook Question
Textbook QuestionThe reaction of tungsten hexachloride (WCl6) with bismuth gives hexatungsten dodecachloride (W6Cl12). WCl6 + Bi ---> W6Cl12 + BiCl3 Unbalanced (c) When 228 g of WCl6 react with 175 g of Bi, how much W6Cl12 is formed based on the limiting reactant?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
511
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos