3. Chemical Reactions
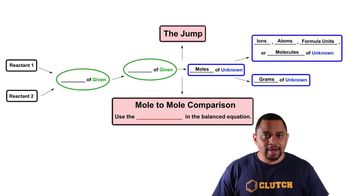
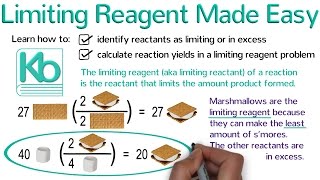
Limiting Reagent
Problem 80a
Textbook Question
Textbook QuestionOne of the steps in the commercial process for converting ammonia to nitric acid is the conversion of NH3 to NO: 4 NH31g2 + 5 O21g2¡4 NO1g2 + 6 H2O1g2 In a certain experiment, 2.00 g of NH3 reacts with 2.50 g of O2. (c) How many grams of the excess reactant remain after the limiting reactant is completely consumed?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
518
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos