3. Chemical Reactions
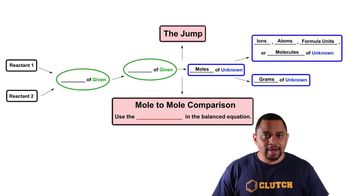
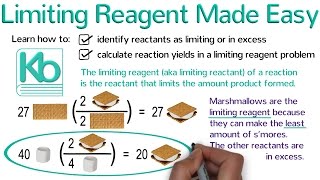
Limiting Reagent
Problem 9
Textbook Question
Textbook QuestionIf 2.00 moles of nitrogen and 5.50 moles of hydrogen are placed in a reaction vessel and react to form ammonia, what is the theoretical yield of ammonia (NH3)? (LO 3.8) N2(g) + 3 H2(g) --> 2 NH3(g) (a) 31.2 g (b) 62.3 g (c) 93.7 g (d) 34.1
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
815
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos