3. Chemical Reactions
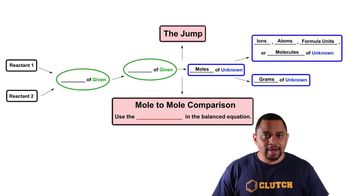
Limiting Reagent
Problem 80a
Textbook Question
Textbook QuestionOne of the steps in the commercial process for converting ammonia to nitric acid is the conversion of NH3 to NO: 4 NH31g2 + 5 O21g2¡4 NO1g2 + 6 H2O1g2 In a certain experiment, 2.00 g of NH3 reacts with 2.50 g of O2. (a) Which is the limiting reactant?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
556
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos