3. Chemical Reactions
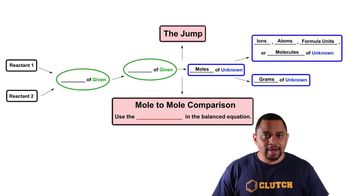
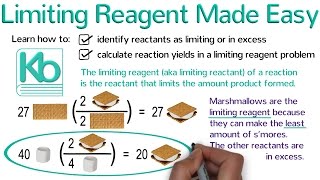
Limiting Reagent
Problem 126
Textbook Question
Textbook QuestionGaseous iodine pentafluoride, IF5, can be prepared by the reaction of solid iodine and gaseous fluorine: I21s2 + 5 F21g2¡2 IF51g2 A 5.00-L flask containing 10.0 g of I2 is charged with 10.0 g of F2, and the reaction proceeds until one of the reagents is completely consumed. After the reaction is complete, the temperature in the flask is 125 °C. (d) What is the total mass of reactants and products in the flask?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
1338
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos