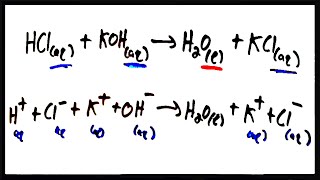6. Chemical Quantities & Aqueous Reactions
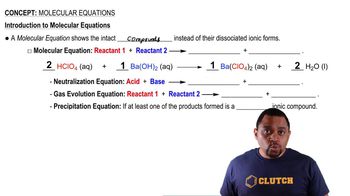
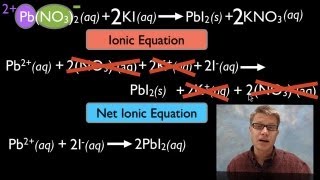
Molecular Equations
Problem 92b
Textbook Question
Textbook QuestionA 25.0-mL sample of 0.050 M barium nitrate solution was mixed with 25.0 mL of 0.050 M sodium sulfate solution labeled with radioactive sulfur-35. The activity of the initial sodium sulfate solution was 1.22 * 106 Bq>mL. After the resultant precipitate was removed by filtration, the remaining filtrate was found to have an activity of 250 Bq/mL. (a) Write a balanced chemical equation for the reaction that occurred.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
445
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos