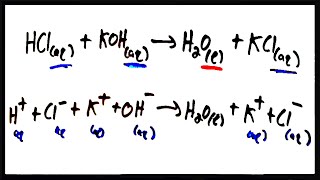6. Chemical Quantities & Aqueous Reactions
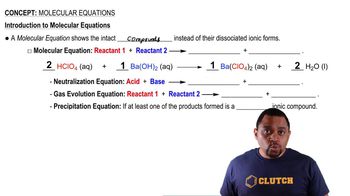
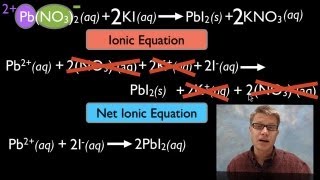
Molecular Equations
Problem 77a
Textbook Question
Textbook QuestionA solution containing several metal ions is treated with dilute HCl; no precipitate forms. The pH is adjusted to about 1, and H2S is bubbled through. Again, no precipitate forms. The pH of the solution is then adjusted to about 8. Again, H2S is bubbled through. This time a precipitate forms. The filtrate from this solution is treated with 1NH422HPO4. No precipitate forms. Which of these metal cations are either possibly present or definitely absent: Al3 +, Na +, Ag +, Mg2 +?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
617
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos