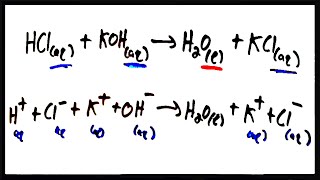6. Chemical Quantities & Aqueous Reactions
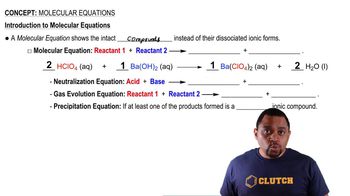
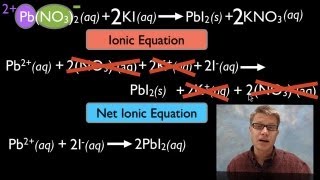
Molecular Equations
Problem 93
Textbook Question
Textbook QuestionA 250.0 g sample of a white solid is known to be a mixture of KNO3, BaCl2, and NaCl. When 100.0 g of this mixture is dis-solved in water and allowed to react with excess H2SO4, 67.3 g of a white precipitate is collected. When the remaining 150.0 g of the mixture is dissolved in water and allowed to react with excess AgNO3, 197.6 g of a second precipitate is collected. (a) What are the formulas of the two precipitates?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
456
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos