In the study of monosaccharides, understanding the process of weak oxidation is essential. Monosaccharides, which are polyols with a carbonyl group, can undergo various oxidation and reduction reactions due to their reactive functional groups, including alcohols and aldehydes. Weak oxidation specifically refers to the selective oxidation of the aldehyde group in monosaccharides to form a carboxylic acid, known as an aldonic acid.
This reaction is particularly significant because it does not affect other functional groups present in the monosaccharide. For instance, while a strong oxidizing agent could oxidize multiple hydroxyl groups to ketones or carboxylic acids, weak oxidation targets only the aldehyde. This selectivity is crucial for differentiating between aldoses (which contain aldehydes) and ketoses (which do not), as weak oxidation will not react with ketoses.
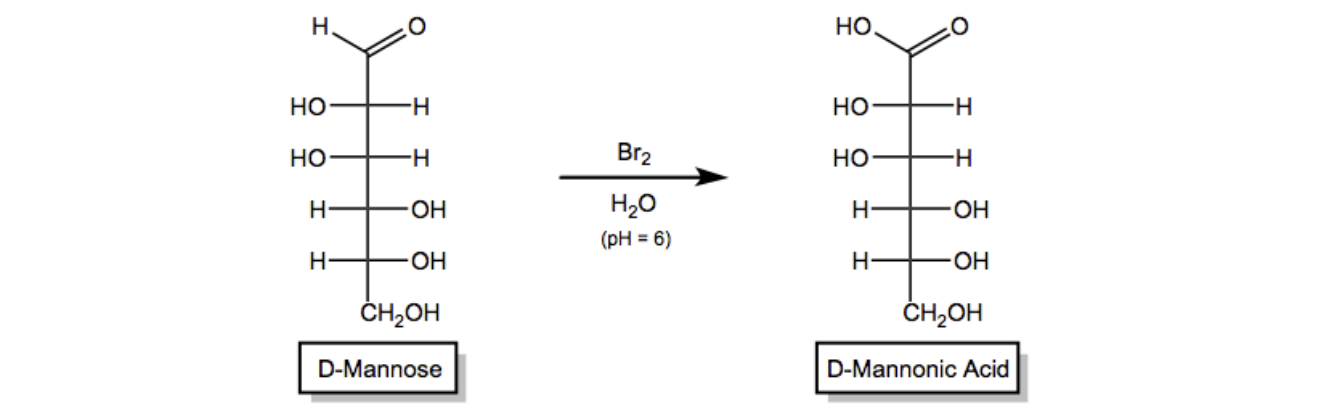
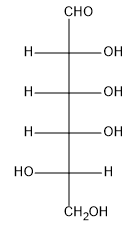
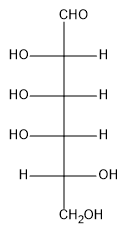
The reagent commonly used for this weak oxidation is bromine water, which is bromine dissolved in water, typically under mildly acidic conditions. This method serves as a basis for certain sugar tests that help identify the type of sugar present. For example, when D-mannose, a common aldose, is treated with bromine water, the aldehyde group is oxidized to a carboxylic acid, while the other hydroxyl groups remain unchanged. The resulting compound is referred to as an aldonic acid.
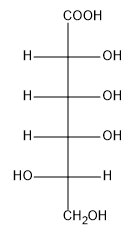
To name the product, the root name of the monosaccharide is combined with the suffix "-onic acid." Therefore, the aldonic acid derived from D-mannose is called D-mannonic acid, indicating that the aldehyde has been oxidized to a carboxylic acid. This transformation highlights the importance of weak oxidation in carbohydrate chemistry and its application in identifying different types of sugars.