Constitutional isomers, also called structural isomers, are compounds that share the same molecular formula but have different atomic connectivity.
Overview:
What exactly is a constitutional isomer? Let’s say you’re given the molecular formula C5H12. Ignoring stereochemistry, how many different compounds can we draw using bondline?

Pentane isomers
We can actually only draw three. Notice that all we’re really doing with carbon-only compounds is changing the branching patterns. If we try to draw any others, we’ll get duplicates or have the wrong number of atoms. Let’s see how different connectivity patterns can give us different functional groups. Let’s draw all the structures for C4H10O:
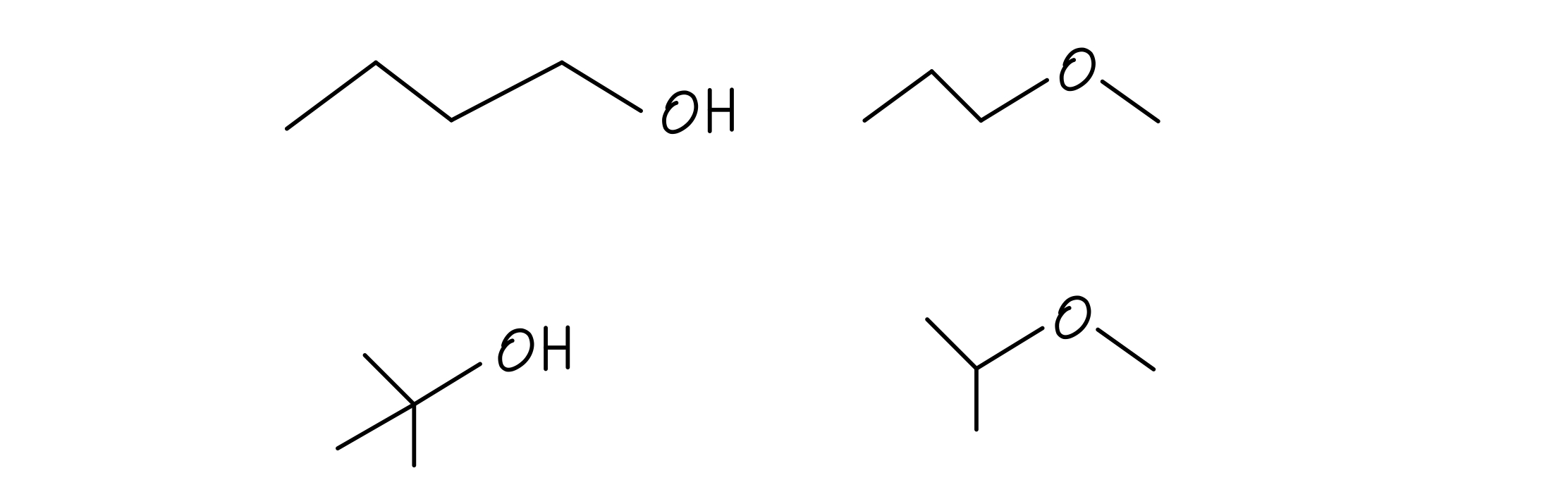
Butanol isomers
Check it out! There are two alcohols and two ethers. That difference in bonding pattern results in the alcohol compounds’ relatively high acidity relative to the ethers. Unfortunately, there’s no systematic way to tell exactly how many constitutional isomers exist just based on a chemical formula.
When drawing isomers of a molecule, chemical formula is key! An easy way to tell the chemical formula of a molecule is to check for rings and pi-bonds. Basically, a ring means that the molecule has one degree of unsaturation, and each pi-bond also means that the molecule has one degree of unsaturation.
Constitutional isomers vs conformational isomers:
Constitutional isomers have different atomic connectivity irrespective of bond rotation, and conformational isomers have the same atomic connectivity and different bond rotation. Let’s see if we can tell the difference:

Chair conformations
Notice that the alcohol and chlorine are on adjacent carbons. The alcohol is facing down on both molecules, and the chlorine is facing up. It might help to convert them to planar structure:

Identical molecules
Okay, so the alcohol is on dash and the chlorine is on wedge. The alcohol's position is also counterclockwise to the chlorine's position, so these are actually the same molecule just rotated.
Let's try to figure out the relationship between molecules in another example:

Chair example 2
Okay, so the alcohol is again counterclockwise to the chlorine, and the alcohol points down while the chlorine points up. Is it the same molecule just rotated again? Nope! Let's find the difference that might be easy to miss.

Constitutional isomers
Check it out! There's a whole carbon between the alcohol and chlorine. These are actually constitutional isomers because they have the same formula but different atomic connectivity.
To be clear, this is not the only form of isomerism. This post only deals with rotation and different atomic connectivity, but there are forms of isomerism that deal with spatial arrangement. Molecules with the same chemical formula and atomic connectivity but different spatial arrangement are called stereoisomers. Some of these stereoisomers (e.g. diastereomers) can actually even have different boiling points.












