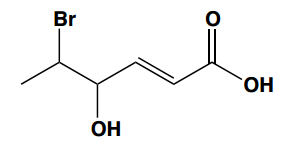
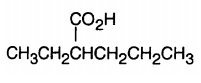Understanding carboxylic acid nomenclature is essential for studying organic chemistry. There are two primary naming systems: the IUPAC system and the common naming system, each with its own advantages.
In the IUPAC system, carboxylic acids are named by modifying the root alkane name. Specifically, the suffix "-e" is replaced with "-oic acid." For example, the root name for a two-carbon alkane (ethane) becomes ethanoic acid. When naming, substituents are indicated using numbers, such as "2-methyl" for a methyl group on the second carbon.
Conversely, the common naming system is rooted in historical usage, as carboxylic acids were among the first organic compounds studied. This system often uses names that do not follow IUPAC conventions. For instance, while ethanoic acid is the IUPAC name, it is more commonly known as acetic acid. To effectively use the common naming system, it is important to memorize the first five carboxylic acids based on the number of carbon atoms:
- 1 carbon: Formic acid
- 2 carbons: Acetic acid
- 3 carbons: Propionic acid
- 4 carbons: Butyric acid
- 5 carbons: Valeric acid
In the common naming system, substituents are not numbered but are instead designated using Greek letters: alpha (α), beta (β), gamma (γ), delta (δ), and epsilon (ε). This approach is unique to common names and differs from the systematic IUPAC method.
Additionally, when dealing with the anionic form of carboxylic acids, the suffix "-oic acid" is replaced with "-ate." For example, acetic acid becomes acetate when it loses a proton and forms a negatively charged species.
In summary, mastering both the IUPAC and common naming systems for carboxylic acids is crucial for effective communication in organic chemistry. Familiarity with the first five common names and the use of Greek letters for substituents will enhance your understanding and application of these concepts in practice problems.