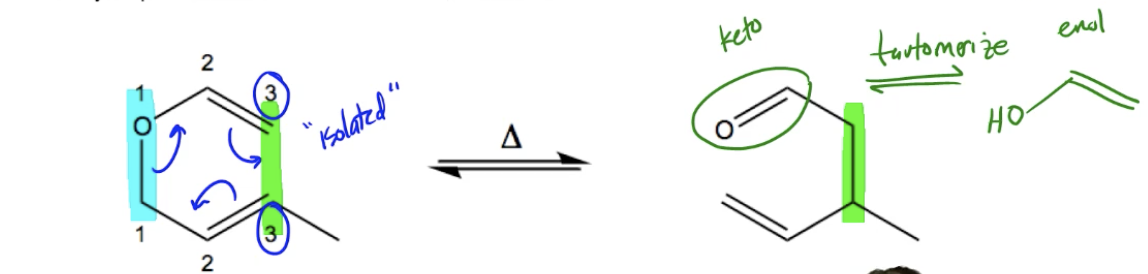
The Claisen rearrangement is a specific type of sigmatropic shift, classified as a 3,3-sigmatropic shift, that requires heat activation and involves an allyl ether as a key reactant. This reaction is unique among pericyclic reactions because it does not initiate with any form of conjugation, allowing it to occur with isolated dienes. The presence of an allyl ether is crucial; without it, the reaction cannot be classified as a Claisen rearrangement.
To identify an allyl ether, it is important to understand that an ether is characterized by the general structure R-O-R, where R represents hydrocarbon groups. An allyl ether specifically has the oxygen atom connected to a carbon that is one bond away from a double bond, forming an allyl group. This structural requirement is the defining feature of the Claisen rearrangement.
In the context of the reaction, the mechanism involves breaking a bond between the first two carbons and forming a new bond between the third carbons, which is why it is categorized as a 3,3-sigmatropic shift. The general mechanism is concerted and pericyclic, meaning that bond formation and bond breaking occur simultaneously in a cyclic manner.
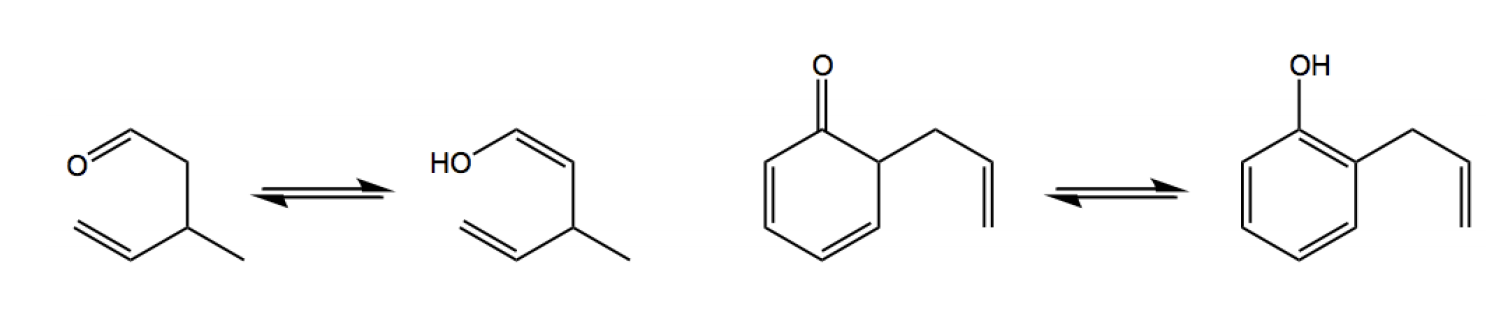
After the Claisen rearrangement, the product often contains a carbonyl group, which can undergo tautomerization to form an enol. Tautomerization is the process where a keto form (the carbonyl) converts to an enol form (where a hydrogen atom shifts and a double bond is formed). While the keto form is typically favored in most cases, certain molecules may prefer the enol form. In such instances, it is essential to recognize the favored tautomer at equilibrium, as failing to do so could lead to incorrect conclusions about the product.
In summary, the Claisen rearrangement is a heat-activated 3,3-sigmatropic shift involving allyl ethers, characterized by its unique mechanism and the potential for tautomerization of the resulting carbonyl product. Understanding these concepts is crucial for accurately predicting the outcomes of reactions involving Claisen rearrangements.